Molecular Targeting
Visualization of molecular events in diseased cells is critical for both advancing basic science discoveries and personalizing treatment regimens for patients. Until recently, molecular events have been monitored predominantly through histological analysis of biopsied tissue. While this approach is accurate, it requires surgery, and the process of mounting, staining, and analyzing histological samples is time consuming. Molecular targeting using nanoparticles offers a way to probe critical molecular events in real time, non-invasively in an in vivo setting.
Nanoparticles as Molecular Probes
Nanoparticles are excellent contrast agents. When used in conjunction with biomedical imaging devices, they can provide bright, enhanced signals with significant differentiation from native tissue background. In certain applications, such as identifying sentinel lymph nodes, stable nanoparticles with passive coatings are sufficient.
However, in many biomedical applications such as cancer and atherosclerosis, it is a sought after goal to visualize molecular perturbations, characteristics, or changes in the tissue in real time. Nanoparticles with active coatings provide a means to probe and visualize these molecular events.
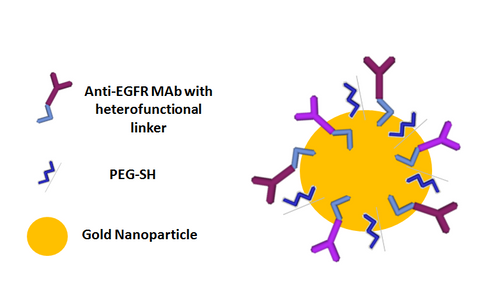
Examples of active coatings include antibodies, folates, peptides, and DNA that can actively bind to or interact with extracellular or intracellular markers. For instance, cancer cells often have specific proteins that are overexpressed on the cell surface. One such protein, epidermal growth factor receptor (EGFR), is overexpressed in certain strains of breast and many other cancers. Nanoparticles can be made to target EGFR by binding the antibody to EGFR directly to the particle (see schematic below).
When these EGFR targeted nanoparticles are in the vicinity of a tumor, they can bind with EGFR and accumulate in and on the surface of cancerous cells that overexpress that marker. The accumulation of nanoparticles in tumors can be visualized with techniques such as photoacoustics to show, in real time, cancer cells that overexpress that marker.
Molecular Targeting In Vitro
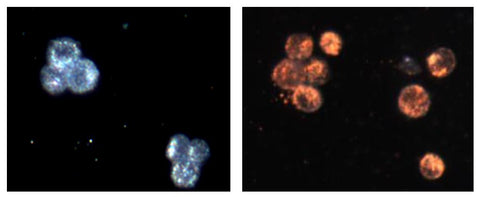
The targeting effect is also dramatic in vitro as well. The image on the left below is a darkfield microscopy image of cancer cells that overexpress EGFR. The image on the right is of these same cells mixed with gold nanoparticles that are targeted to EGFR. The change in color of the cells is due to massive aggregation of gold on the surface and in vesicles called endosomes inside the cells.
Examples of other antibodies that can be used in a similar fashion to target cells include the antibodies against Human Epidermal Growth Factor Receptor (HER2, Trastuzumab, or Herceptin), Clusters of Differentiation (CD24, CD33, or CD45), Epithelial Cell Adhesion Molecule (EpCAM), or integrins (alpha-v beta-3) etc. It is exciting that nanoparticles can probe cancer cells and label diseased cells preferentially allowing for diseased states to be visualized both in vitro and in vivo.
Active Nanoparticle Coatings to Enable Molecular Imaging
NanoHybrids currently provides passively coated gold nanosphere and gold nanorod products. Passive coatings include polymer layers such as polyethylene glycol (PEG) or silica. For silica-coated particles, the functional hydroxyl group or silane chemistries can be used to conjugate active targeting molecules to the surface. Traditional antibody conjugation kits for gold nanoparticles will not work for our silica coated particle collection, so please contact us if you want advice or more information about performing these bioconjugations on your own.
Antibody Conjugated Nanoparticles
In 2014 NanoHybrids will be introducing new lines of gold nanoparticle products to help researchers conjugate their molecule of choice to the nanoparticle surface. NanoHybrids will offer surface functional groups such as amines, thiols, maleimides, and carboxylic acid to act as handles that enable a myriad of bioconjugation methods. Furthermore, we will also introduce antibody conjugated nanoparticles that are sterile and ready for use in vitro and in vivo.
NanoHybrids has developed a proprietary bioconjugation method for directional attachment of antibodies to nanoparticles that significantly enhances cell labeling efficiency. Many researchers find that while performing conjugations on their own, they suffer from inconsistent antibody to particle attachment and irreproducible cell labeling efficiencies. NanoHybrids’ directional antibody attachment methods lead to well characterized antibody to particle ratios. Furthermore, since the variable domain region (Fv) of the antibody is never sterically hindered, cell labeling is more efficient and consistent batch to batch.
Contact us today for your custom antibody conjugation requirements.
Related Links:
Gold Nanoparticles & Antibody Conjugation
Applications of Gold Nanoparticles
Gold Nanoparticles Designed for Lateral Flow Assays
Photoacoustic Molecular Imaging with nanoparticle contrast agents